170
of some studies are still not available) in order to study
and compare several therapeutic modalities, of which
the following should be highlighted:
- Anti-Vascular endothelial growth factor (VEGF)
Antibody for the Treatment of Predominantly Classic
Choroidal Neovascularization (CNV) in AMD (Anchor
Study
(16,17,18)
)
- Ranibizumab
Combined
with
Verteporfin
PhotodynamicTherapy in Neovascular AMD (Focus
(19)
)
- Summit Clinical Trial Program, which includes 3
studies: the Mont Blanc, Denali and Everest Studies
(20)
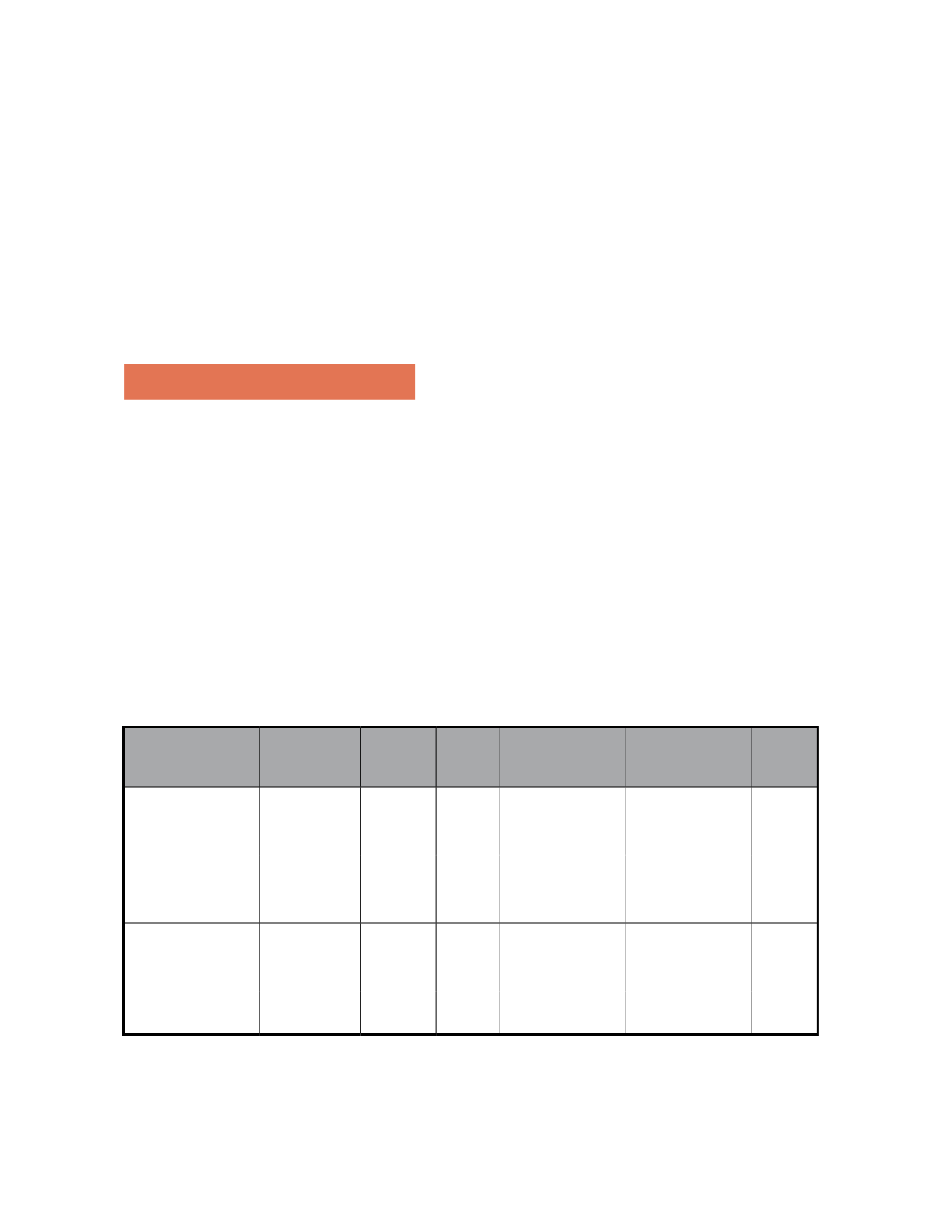
3.1 TAP Study(Table 1)
This study provided the main evidence of PDT effi-
cacy. It included two multicentric, double-blind, ran-
domized, placebo-controlled studies, in Europe and
the United States of America. Four hundred and two
patients with classic subfoveal choroidal neovascu-
larization were treated with PDT, while 207 patients
were treated with placebo. The primary endpoint was
the percentage of eyes for which losses of less than 15
ETDRS letters from baseline were observed at 12 and
24 months.
PDT was significantly more effective than the placebo,
both at 12 months (61% versus 46%) and 24 months
(53% versus 38%). These results were more significant
in predominantly classic membranes.
regime
(4)
. No statistically significant differences were
found between the two regimes in terms of visual
improvement, number of retreatments and safety. The
intensive treatment regime in the first 6 months appears
to be more effective in preventing severe loss of visual
acuity; however, the difference observed after 24 months
is not statistically significant, with loss of visual acuity
greater than 6 lines being observed in 25% of patients
treated with the intensive regime and 38% of patients
treated with the standard regime.
3. Main clinical trials
The efficacy of PDT was evaluated in several multicen-
tric, randomized clinical trials in patients with AMD
with choroidal neovascularization, of which the follow-
ing should be highlighted:
- Treatment of AMD with PDT (TAP studies)
(5,6,7,8,9)
- Verteporfin in PDT (VIP studies)
(10,11)
- Verteporfin in Minimally Classic Choroidal
Neovascularization (VIM studies)
(12)
- Visudyne
in
Occult
Classic
Choroidal
Neovascularization (VIO study)
(13)
- Meta-analysis of the TAP and VIP Studies
(14)
- Treatment of AMD with PDT – 5-year extension
Study - TAP Extension
(15)
Many studies were subsequently performed (the results
Study Number of patients
Pred. classic
Verteporfin
Pred. classic
Placebo
p
All membranes
Verteporfin
All membranes
Placebo
p
TAP: 12 months
N=609
67.3%
39.8% <0.001
61.2%
46.4%
<0.001
TAP: 24 months
N=609
59.1%
31.3% <0.001
53%
37.7%
<0.001
TAP: 36 months
N=476
58.1%
---
TAP: 48 months
57%
---
Table 1- TAP study: percentage of eyes with loss <3 lines in the ETDRS chart


