171
Photodynamic Therapy
3.2 VIP Study (Table 2)
In this study, the efficacy and safety of Photodynamic
Therapy were evaluated in patients with occult lesions.
Results after 12 months were somewhat disappointing;
however, efficacy was demonstrated in the treated group
at 24 months (46.2% versus 33.3%).
Subgroup analysis led to the conclusion that greater ben-
efits were achieved in patients with small lesions (less
than 4 disc areas) and/or visual acuity worse than 20/50.
In these patient subgroups, the differences between the
PDT group and the placebo group had greater statistical
significance (51% versus 25%).
3.3 VIM Study (Table 2)
The objective of this study was to determine the efficacy
of photodynamic therapy in minimally classic mem-
branes (where the classic component represents less than
50% of the neovascular lesion) sized below six disc areas.
Additionally, the efficacy of reducing fluence to 50%
(25J/cm
2
) relatively to standard parameters (50J/cm
2
)
was also analysed. In the standard laser light activation
protocol, a wavelength of 689 nm and an intensity of
600 mw/cm
2
are used for 83 seconds to achieve a flu-
ence value of 50J/cm
2
. In this study, no statistically sig-
nificant efficacy was found at 12 and 24 months in the
group of patients treated with the standard protocol. On
the contrary, better results were observed for patients
treated with the reduced fluence protocol, in terms of
the primary endpoint (loss of visual acuity of less than 15
letters). Based on these results, the study authors advise
treatment of small minimally classic lesions with PDT,
concluding that the reduced fluence protocol may be
beneficial. The percentage of conversion of minimally
classic lesions into predominantly classic lesions was also
studied and treatment efficacy was demonstrated, irre-
spective of the fluence used.The reduced fluence issue
will also be referred in the Denali study. This study
investigates the efficacy and safety of combined therapy
involving PDT and antiangiogenic drugs, namely ranibi-
zumab 0.5 mg, administered intravitreally. Patients were
randomized to receive intravitreal injections of ranibi-
zumab 0.5 mg, in monotherapy or combined with PDT,
with standard or reduced fluence. This study, which
started in May 2007, includes 321 patients and is cur-
rently in course in the United States and Canada. The
results of the Denali study are not yet available.Two
other studies – VALIO (Verteporfin Therapy with Altered
Light in Occult choroidal neovascularization) and VER
(Verteporfin Early Retreatments) were also performed. In
the VALIO study, the efficacy of laser treatment at 15
and 30 minutes was evaluated and compared. Since no
statistically significant differences were observed between
these two therapeutic modalities, it was decided to main-
tain the 15 minutes used in standard treatment. The
objective of the VER study was to determine whether
it would be beneficial to reduce treatment intervals to 6
weeks in the first 6 months. Since no increase in efficacy
was found relatively to the standard regime (treatment
every 3 months), it was advised that the usual treatment
regime be maintained.
3.4 VIO Study
The VIO study was designed to determine PDT indi-
cations in occult lesions with no classic component.
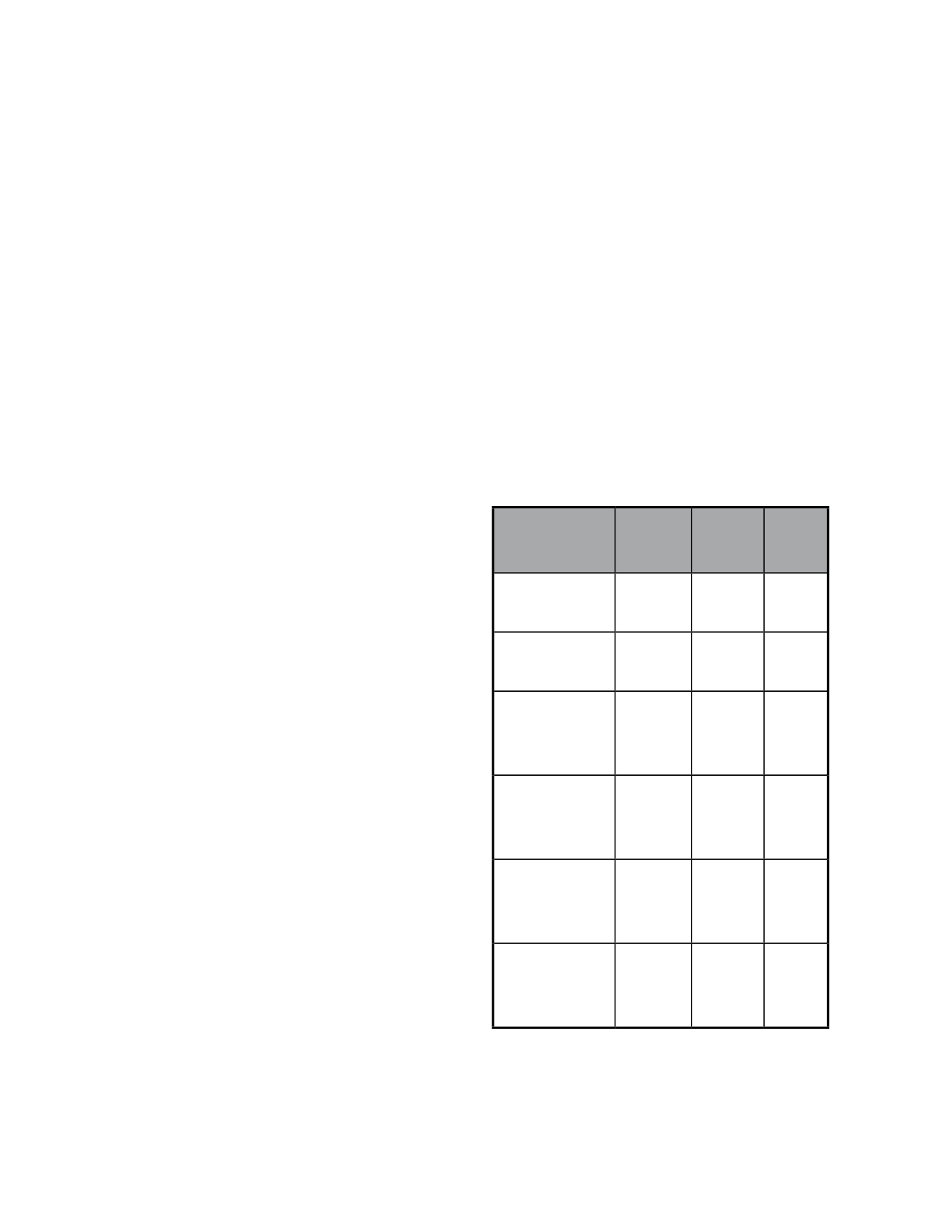
Study
MTRI
verteporfin
MTRI
Placebo
P
VIP 12 months
49%
45%
Ns
VIP 24 months
45%
32% 0.032
VIM 12 months
300 mw/cm
2
86%
53% 0.002
VIM 12 Months
600 mw/cm
2
72%
53%
0.08
VIM 24 months
300 mwcm
2
74%
38% 0.003
VIM 24 months
600 mwcm
2
47%
38%
0.45
Table 2- VIP and VIM studies: percentage of eyes with loss <3 lines in
the ETDRS chart.


