102
ments as lipofuscin that accumulate in RPE cells,
contribute to a decline of the cell function and
degeneration conducting to GA
(27)
.
Although RPE cells are the most involved, the outer
nuclear layer is also severely affected with dysfunc-
tion and death of photoreceptors. Probably rods are
the first affected photoreceptors
(31,32)
.
The mechanisms of RPE death are best studied at
junctional zone. Here, lipofuscin may occupy 30%
of RPE cell and may interfere with its metabolism,
conducting to death. These mechanisms include oxi-
dative stress and inflammation
(33,34,35,36)
.
Macrophages are often seen in areas of GA, appar-
ently phagocytosing pigment and debris resulting of
normal cells deletion
(37)
.
5. Diagnosis
5.1 Fundus
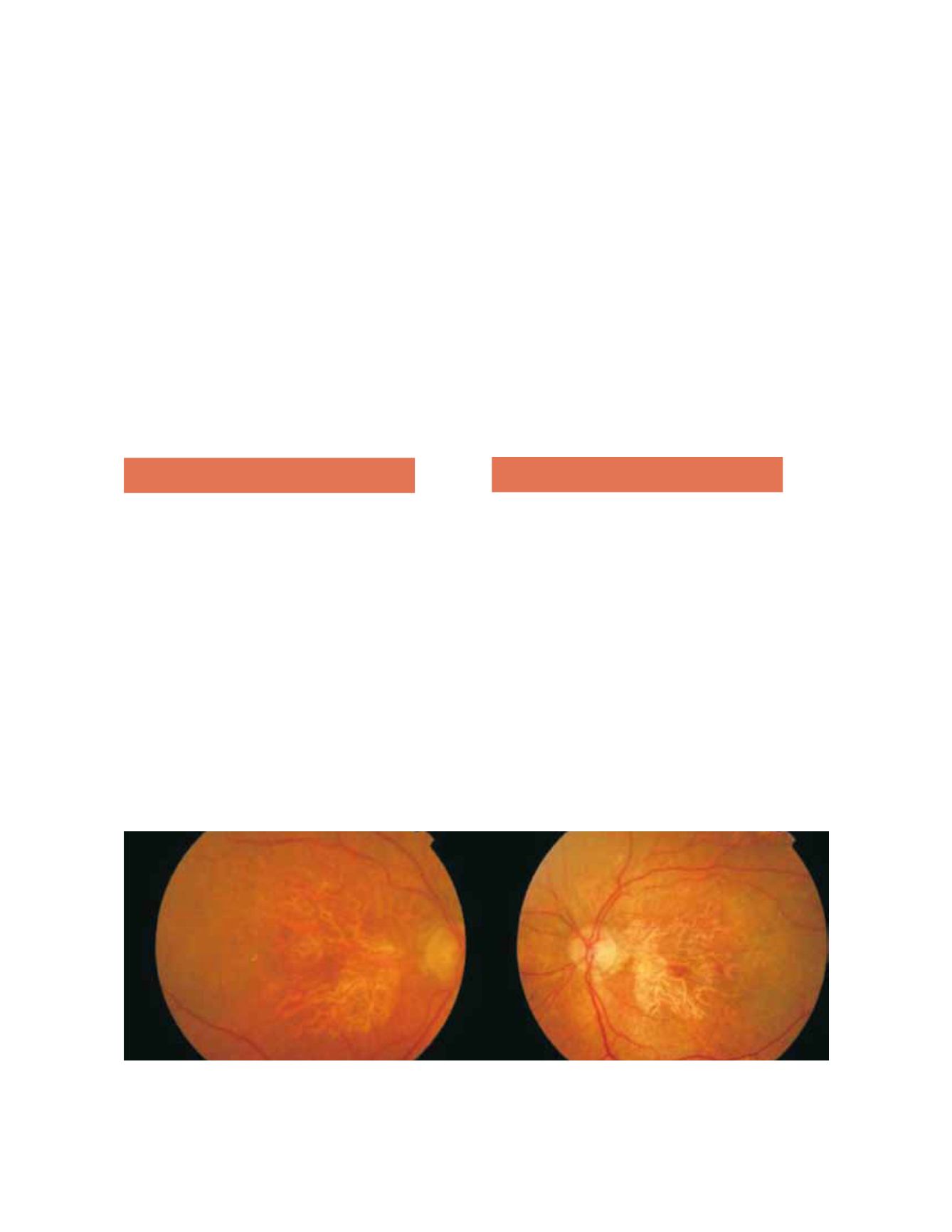
Fundoscopy in GA typically shows a well-circum-
scribed oval or round area of pigment epithelium
atrophy, usually sparing the fovea until late stages
(Fig. 2). All precursor lesions of this final appearance
can also be present: large drusen (>125 microns),
focal pigmentation changes and refractile depos-
its
(3,23)
.
5.2 Angiography
On fluorescein angiography, GA appears as a sharply
delineated window defect due to atrophy of overlying
layers of RPE (Fig. 3).
A prolonged choroid filling phase has been described
(2.1% vs 4.8%) like in exudative forms
(14,15)
.
In Rotterdam and Beaver Dam Eye Study, serum
HDL cholesterol was directly associated with GA,
however this association was not found in Blue
Mountains Eye Study
(16)
. In this study, diabetes
and the ratio total/HDL cholesterol were linked to
increased risk of GA
(18)
.
Genetic risk factors have been described in associa-
tion with AMD. As complement system seems to
play an essential role in this disease, the complement
factor H (CFH) gene located at chromosome 1q32,
and others as CFB, LOC HTrA1, C2 and C3, have
been implicated in the development of both forms of
AMD
(18-20)
. Some studies have linked specifically 5p
region and 4q 32 region with GA
(21,22)
.
4. Pathology
Accordingly to AREDS the most common sequence
of events leading to GA is the progression of a large
drusen to hyperpigmentation, followed by regression
of the drusen, hypopigmentation and ultimately RPE
cell death, with development of an atrophic area of
retina and underlying choriocapillaris, sometimes
preceded by the appearance of refractile deposits.
This evolution can be longer than 6 years
(3,23,24)
.
Less frequently, GA can follow a drusenoid RPE
detachment, regression of a CNV membrane or a
RPE rupture
(5,25,26)
. In some eyes, atrophy was related
to a micro reticular pigment pattern distributed
around the perimeter of the fovea
(26)
.
Most histopathologic studies suggest that RPE cells
are the primary target in GA and its death results
in choriocapillaris atrophy
(29,30)
. Autofluorescent pig-
Figure 2. Fundus photography showing a well-circumscribed round area of pigment epithelium atrophy, sparing the fovea in right and left
eyes.


